The Nanotheranostics project – NT1 is the first and main program carried by TORSKAL. It relates to the treatment of skin cancers by photothermal therapy. The product is a class 3 medical device, consisting of gold nanoparticles designed by green chemistry which is planned to undergo clinical trials in 2022.
Skin cancer is one of the most common cancers worldwide. By the age of 70, 20% of Americans will be diagnosed of skin cancer leading to a total treatment cost of $8.1 billion per year only in the US. It is characterized by an abnormal growth of skin cells, most often on skin exposed to the sun. Basal cell carcinomas and squamous cell carcinomas are the two main forms of skin cancers and they are usually not lethal as they evolve slowly and almost do not spread. As they occur on sun-exposed skin, surgical treatment of these tumors could lead to visible scars and disfiguration. Melanomas are the third form of skin cancer and the most dangerous as it evolves very quickly and is able to spread to other parts of the body. Hence, it needs to be treated as soon as diagnosed as a matter of one day can change the prognosis. As of now, the 5-years survival rate for an advanced melanoma is below 40%.
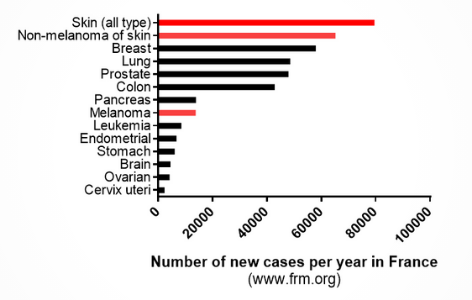
Source: frm.org
Therefore, there is an absolute need not only for new treatment but for new concept, strategies of therapy. Photothermal therapy is one of these new concepts, and we do believe it will help to efficiently treat these diseases with a lower morbidity.
Photothermal therapy consist to use light and a light-sensible “platform” to heat cells. A local temperature increase of 7°C is enough to kill tumor cells. In our case, the light-sensible platform is gold nanoparticles. Using Hubertia ambavilla aqueous extract, an endemic plant from the Reunion Island, as a reducer and a stabilizer, gold nanoparticles are greenly synthetized according a patented process. These gold nanoparticles have the ability to heat when they are excited by a near infra-red laser.
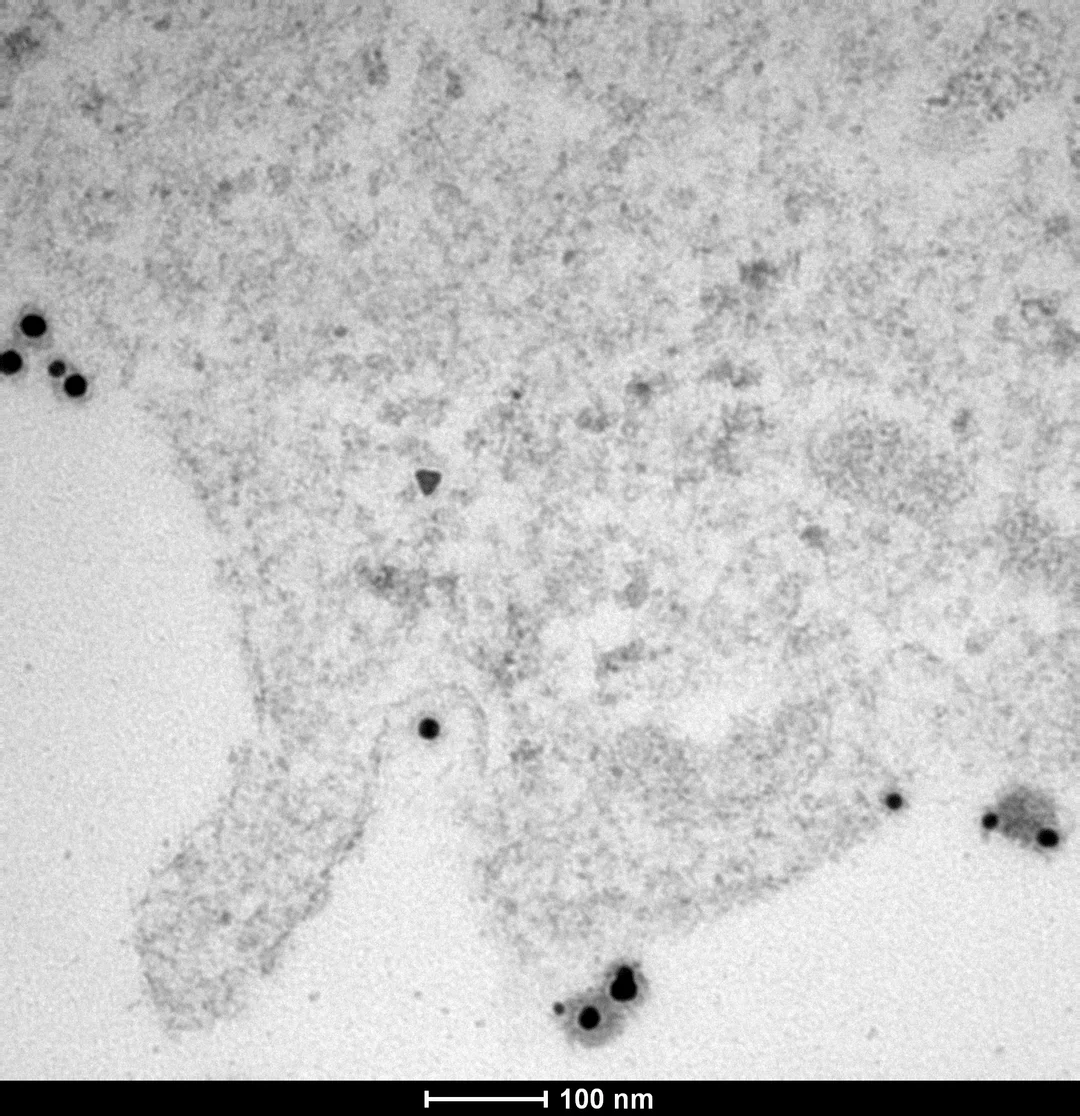
Gold nanoparticles entering a melanoma cell
Using cancerous cells specifiers (metabolism, protein production, etc.), we designed our gold nanoparticle coating in order to optimize their internalization in cancerous cells rather than the healthy ones. Gold nanoparticles are inert for both healthy and cancerous cells without further intervention. Under a near-infrared laser (also inert if used alone), gold nanoparticles will undergo photothermal heating, resulting in selective hypothermic cell death without heating of the adjacent non-tumor tissue. This double targeting (of gold nanoparticles on one side and the laser on the other side) will permit a decrease of side effect in comparison with current strategy.
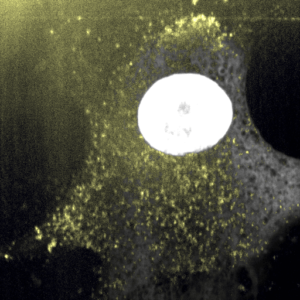
Gold nanoparticles internalization in melanoma cells
NT1 is the most advanced Nanotheranostics project. All the preclinical studies have been validated and the product shows no toxicity at any level and a good anti-tumor activity by inducting hyperthermia in both in vitro and in vivo experiments. Local and systemic administration of the gold nanoparticles have been studied in order to optimize the therapy and has shown that cells die through apoptosis rather than necrosis (which could induce inflammation and acute side effects). PTT using TORSKAL’s gold nanoparticles should be a powerful one single injection therapy with less side effects and a brand new tool in the therapeutical array at the disposal of physicians.
Designing nanovectors using green chemistry is co-financed by the European Union and the Réunion Region (FEDER 1.15). A postdoc was also co-financed on this same research program (FEDER 1.16).
The NT1 project also benefited from funding from MESR (CIR) and BPI France (recoverable advance).
Therapeutic approach:
Scientific partners: LCSNSA (Université de la Réunion), GIP CYROI, Institut Pasteur, Mybiotech, Filab, Université Sorbonne
Patent: 2016 – Gold nanoparticles and ecological preparation process, Patent FR 16/50520, PCT / FR2017 / 050131
